Conformal Medical, Inc.
Developing the next generation of LAAO technology.
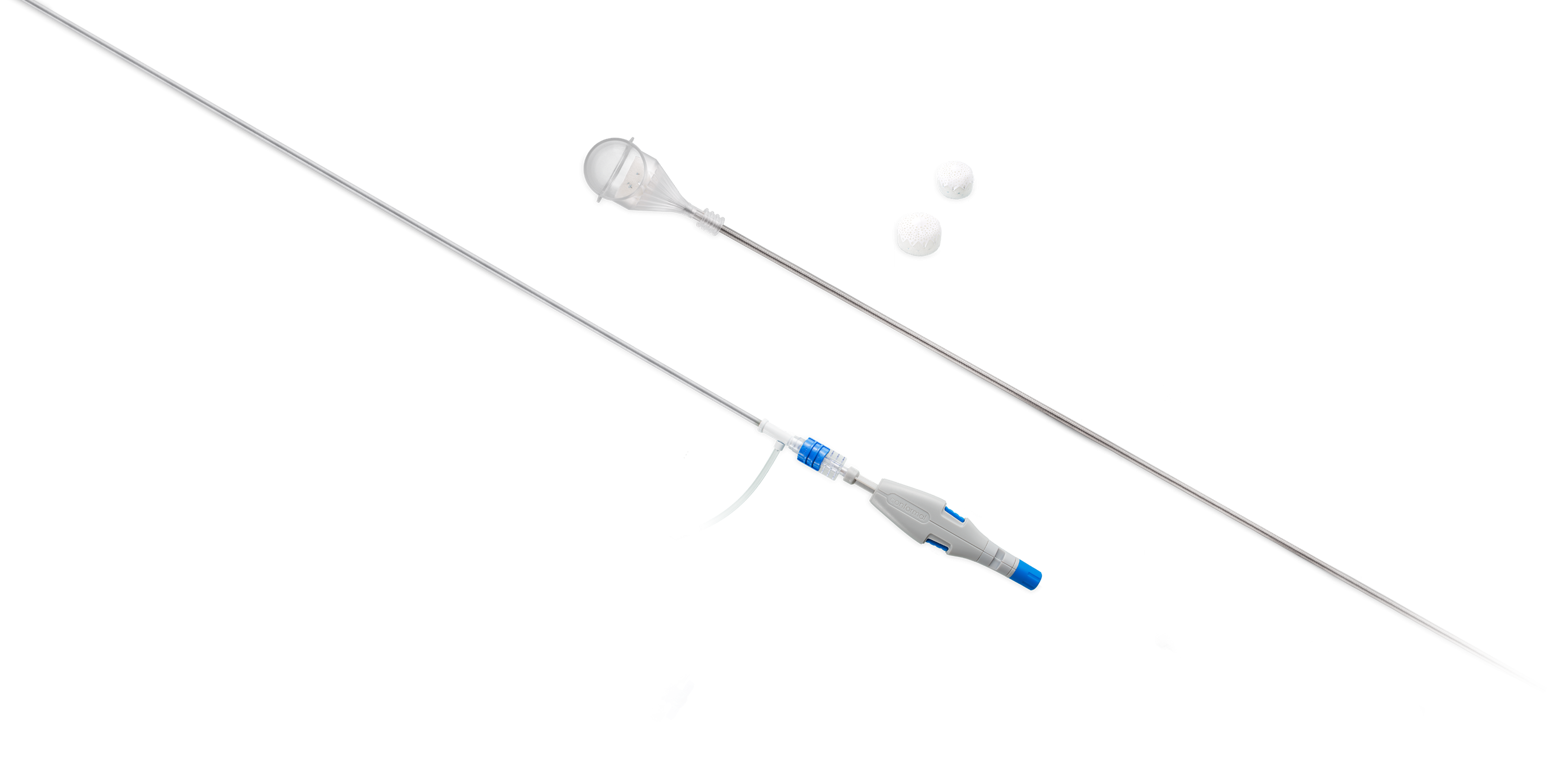
Conformal® is a privately held medical device company that designed the CLAAS System* to seal the LAA to reduce the risk of stroke, without the need for anticoagulants.
*Currently in clinical testing and not available for sale.